In the manufacturing industry, the use of programming codes is essential to automate processes and enable them to operate efficiently at different scales. Within this context, APIs (Application Programming Interfaces) allow software applications to communicate with each other in order to exchange data, resources, and functionalities. These APIs can be designed or implemented using REST or GraphQL architectures and utilize formats such as JSON and XML for data transmission, which are widely adopted for software integration.
Each sector of the industry applies APIs in specific ways: in the pharmaceutical industry, these interfaces can be used to integrate research data, clinical trials, production, and logistics. For vaccine development, they enable access to genomic databases, communication between clinical trial monitoring systems, and digital tracking of raw materials and production batches. This capability ensures agility, security, and control at every stage of the manufacturing process.
Integrating databases and systems, such as bioinformatics, molecular modeling, and artificial intelligence, accelerates preclinical research (the initial stage in the development of any drug, including vaccines). This integration enables the prediction of antigen structures, immunogenicity, toxicity, and efficacy of vaccine candidates. Databases such as GenBank and PubMed (highly utilized during the research phase), provided by the NCBI, are essential for genomic analyses, optimizing critical stages of the pharmaceutical pipeline.
Paulo Moro, MSc in Biosciences and Biotechnology, explains: “[…] the industry leverages knowledge generated by basic research to develop products and optimize processes; this underscores the importance of collaboration between universities and companies.”
In vaccine production and the development of applications for the life sciences sector, APIs can play a key role in facilitating interaction between biological science professionals and the data available in these repositories, whether directly—with the user being a bioinformatician—or as a developer creating an application for use in the biological sciences.
Paulo also highlights: “[…] having an API allows for additional security layers to ensure the integrity of information; moreover, it guarantees that only accurate information is inserted, read, updated, or deleted from the database.”
Pharmaceutical Industry Database
The pharmaceutical industry operates under a stringent set of regulations, particularly regarding the development and manufacturing of vaccines. In the context of large-scale vaccine production, one such regulation involves the use of cryopreserved samples of biological materials, such as cells and viruses.
This stage also requires a high-quality database to efficiently and reliably track and manage these samples. For this process, robust programming validation to structure an effective database is essential, ensuring accuracy, consistency, and reliability across all operations.
To properly cryopreserve a sample, well-defined protocols are followed. Typically, this involves cryopreservation process that reduces the sample’s temperature in a controlled manner. Often, cryoprotectants are used to minimize ice crystal formation that can damage cells. Storage occurs at ultra-low temperatures, such as in liquid nitrogen.
The quality of the sample cryopreservation process has a direct impact on large-scale vaccine manufacturing. Properly preserved samples ensure the reliability of quality control assays, which is essential for regulatory approval and widespread distribution. Any compromise in sample integrity can cause delays in production, jeopardize supply, and affect the efficacy of the final product.
An example of this, as explained by Paulo Moro, is: “[…] in the pharmaceutical industry, cell lines are extensively utilized in research. It is crucial to know the origin and preservation conditions of these cells to ensure the validity of the results.”
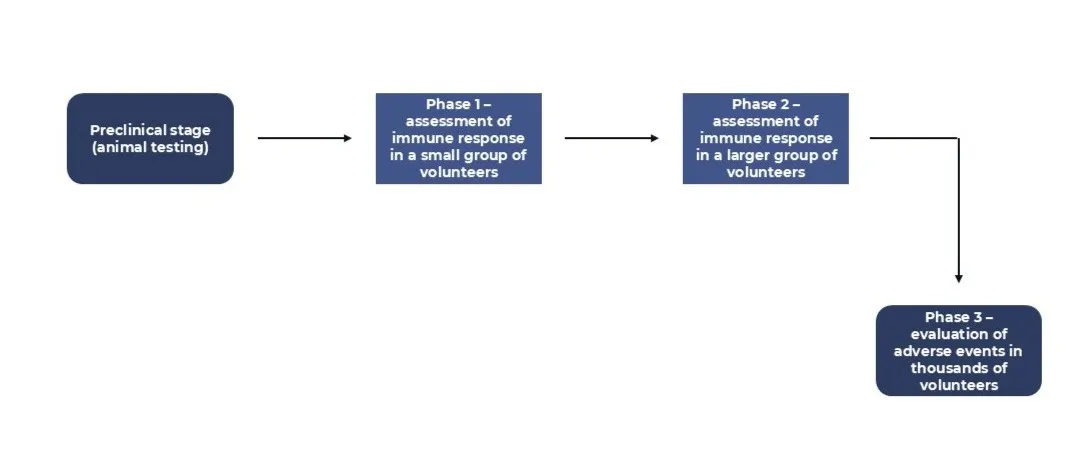
- Preclinical stage: validation of immunogenicity, protective efficacy, and safety;
- Phase 1: evaluation of optimal dosing, safety profile, and vaccine immunogenicity;
- Phase 2: conducted with over 100 participants, continued assessment of safety and immune response;
- Phase 3: the vaccine is administered and compared to a control group receiving a placebo (an inert substance with no therapeutic effect).
Application of APIs in Molecular Weight Analysis for Vaccine Production
Several stages of the vaccine manufacturing process require automated systems with programming capabilities to ensure efficiency, such as antigen cultivation and the purification phase. Paulo emphasized that automation is essential in the pharmaceutical industry to ensure the safety of biologics production processes: “[…] many processes are automated to guarantee precision and consistency, reducing the possibility of human error.”
To illustrate the use of APIs in the pharmaceutical industry, the molecular weighing step will be used as a reference, as it directly impacts the efficacy of a vaccine’s immune response. One possible workflow involves collecting data from publications on PubMed using the NCBI API, known as “Entrez Programming Utilities”.
After extracting the information from the target publication, the data needs to be processed, cleaned (to avoid inconsistencies), and stored in a CSV or JSON file. It’s also possible to visualize the data using libraries such as matplotlib, seaborn, or plotly to generate trend charts.
For this specific simulation, a dataset based on the “HelA [Escherichia phage Tls]” protein, or YP_001285548.1, was used, which can be retrieved using a Python Notebook:
df = pd. DataFrame(dados)
#Generate Molecular Weight Distribution Plot
plt.figure(figsize=(10,5))
plt.bar(df['Sample'], df['Peso_Molecular_kDa'])
#Customize Plot
plt. xlabel('Sample')
plt.ylabel('Molecular Weight (kDa)')
plt. title('Distribuição do Protein Molecular Weight Distribution YP_001285548.1')
plt.grid(axis='y')
plt. show()
Python Code Simulation of HeLa Cell Protein
In this context, leveraging an API aims to automate database queries, such as those to PubMed, using Python scripts developed for this purpose. To visualize the results of this simulation, you can utilize a Python Notebook to display the relationship between the properties of the relevant protein and the distribution of molecular weights:
Molecular Weight Chart for Vaccine Production
Understanding the molecular weight of the antigen in vaccine manufacturing is essential for identifying its physicochemical properties and ensuring quality control. It serves as a critical parameter throughout every stage of production, guaranteeing the safety of the final product.
Learn more about ST-One.